Research
The Nguyen lab is dedicated to translating cutting-edge research into life-changing therapies for patients suffering from cancer, myocardial infarction, colitis, and other diseases. Our interdisciplinary team of experts combines the power of molecular engineering, pharmaceutical sciences, and bioinformatics to develop genetically and molecularly engineered biotherapeutics that are safe, effective, and tailored to the individual needs of each patient.
Our mission is to revolutionize medicine by developing next-generation therapeutics that target diseases at the molecular level, offering hope and improved quality of life to patients and their families. With a relentless focus on translational research and a commitment to the highest standards of scientific excellence, the Nguyen lab is dedicated to making a difference in the lives of patients around the world. Join us in our mission to transform healthcare through innovative biomolecular engineering.
Research Interests
Developing therapeutics for cardiac repair
Coronary heart disease is a global health crisis, claiming millions of lives every year. When a heart attack occurs, a significant number of cardiomyocytes, the specialized muscle cells responsible for contracting the heart, die and are replaced by non-contractile scar tissue. This can lead to a weakened heart and ultimately, heart failure.
In our lab, we are dedicated to changing the course of heart disease by developing innovative biomaterials that can prevent cell death and reprogram different types of cells into functional cardiomyocytes, effectively repairing damaged cardiac tissue and restoring normal heart function.
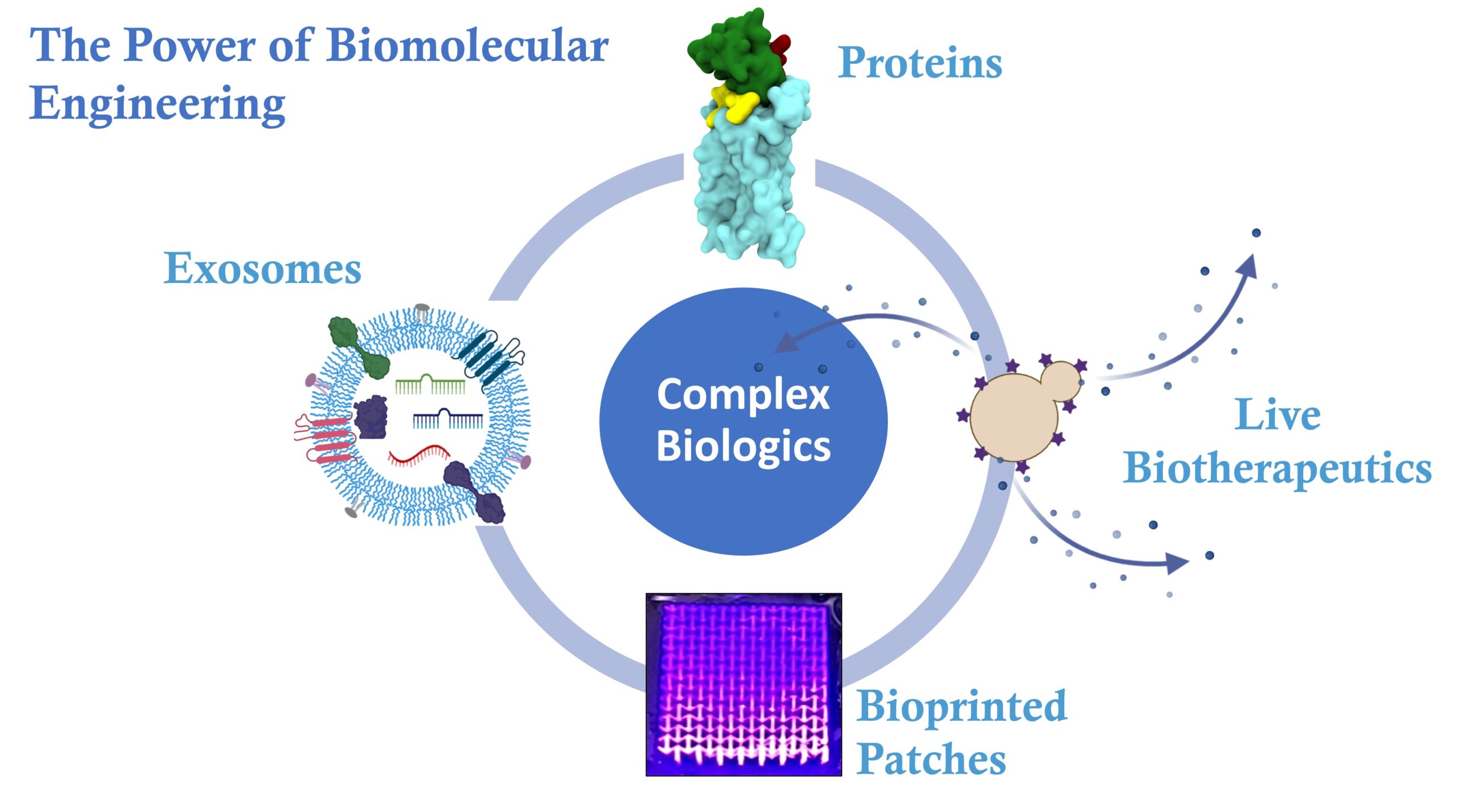
Genetically encoded materials – protein-based materials to target tumor-associated macrophages
While current anticancer therapies primarily target cancer cells, the tumor stroma also plays a crucial role in cancer progression. To address this gap in treatment, we have developed a novel drug delivery approach that targets not only tumor cells but also inflammatory cells. Specifically, we developed a delivery platform that utilizes single-chain variable fragment antibodies to inhibit inflammatory monocyte migration to tumors and polarize macrophages towards the tumoricidal M1 phenotype by hijacking the CCL2/CCR2 pathway. This approach capitalizes on the targeting specificity of antibody fragments and exploits CCR2 receptor structure and function to improve therapeutic efficacy.
Our protein delivery approach minimizes carrier-induced toxicity, as the antibody fragments serve both as a delivery vehicle and therapeutic drug. Through this proposal, we synthesized a modular CCR2-targeting system for macrophage polarization to promote macrophage polarization, and inhibit tumor growth and metastasis in a triple-negative breast cancer (TNBC). Our therapeutic is designed to be used in combination with anti-PD-1 blockade and other therapeutics for the potential treatment of cancer.
Live biotherapeutics for the treatment of inflammatory bowel diseases – the power of engineered, probiotic yeast
Inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis, are chronic and debilitating conditions affecting millions of people worldwide. Current therapies offer only limited relief, leaving patients struggling with severe pain, diarrhea, and other symptoms, often leading to hospitalization and surgery.
In our lab, we are developing the next-generation of therapeutics to treat IBD, using the power of engineered, probiotic yeast to create live biotherapeutics that can effectively reduce inflammation and modulate the gut microbiome towards a protective composition. By focusing on locally acting therapeutics, we can provide a more targeted and effective treatment, while minimizing the risk of systemic side-effects.
Our innovative research is driven by a passion for transforming the lives of IBD patients, who have long been underserved by traditional therapies. By harnessing the power of biomolecular engineering and cutting-edge bioinformatics, we are paving the way towards a future where IBD is no longer a source of suffering, but a manageable condition.
Auxetic patches for dynamic organ repair and wound healing
We developed a new translational patch material with advanced properties that could revolutionize the field of bioadhesive materials and patches for surgical procedures and interventions. This new material exhibits instant adhesion to wet tissues, ultra-stretchability, compatibility with rapid photo-projection, and the ability to deliver therapeutics. Through in silico design and screening more than 100 patches, we created next-generation patches that conform to organ mechanics and instantly attach to a broad range of tissues. In vivo studies have demonstrated remarkable wound healing capability, without the need for removal that can cause pain and bleeding. Additionally, we have developed a new single material-based patch for the treatment of lung puncture wounds.
Funding:
![]()